the building block of matter; indivisible
atom
a bond between two atoms where the atoms share electrons
covalent bond
a substance composed of only one type of atom
element
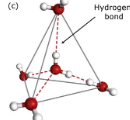
a bond between molecules due to the attraction of slightly negative and slightly positive charged atoms
hydrogen bond
two or more atoms chemically combined
molecule
What kind of bond is this?
covalent bond
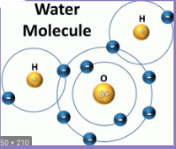
What kind of bond is this?
hydrogen bond
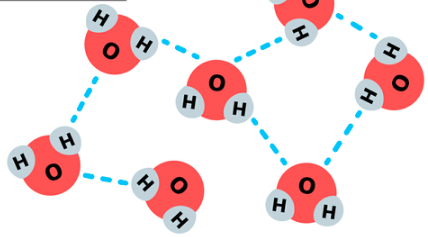
What is water made of?
2 hydrogen atoms & 1 oxygen atom
Why is most water on Earth liquid and not gas?
Because of hydrogen bonds between water molecules.
the phase change from a liquid to a solid
freezing
the phase change from a solid to a liquid
melting
the phase change from a liquid to a gas
vaporization
What phase of matter of water has the following properties:
ice
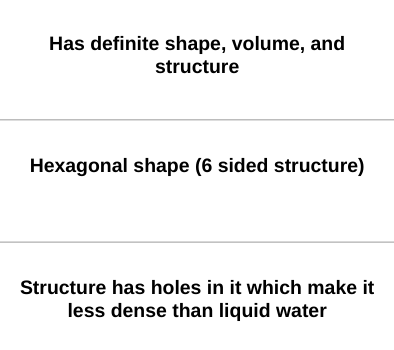
What phase of matter of water has the following properties:
liquid water
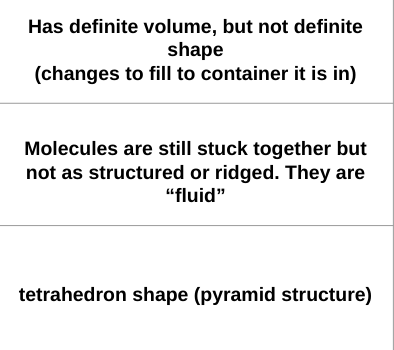
What phase of matter of water has the following properties:
gaseous water

What phase of water has a hexagonal shape caused by their hydrogen bonds?
Ice has a hexagonal shape
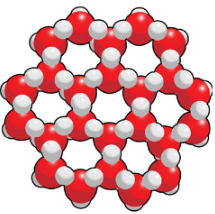
What phase of water has a tetrahedron shape caused by their hydrogen bonds?
liquid water
How does higher salt content (salinity) in water effect the boiling point of water.
High salinity increases the boiling point of water
the amount of energy needed to raise the temperature of a substance by 1 °C
heat capacity
mass per unit volume
density
How much water must an object displace to be able to float?
An object needs to displace as much water as it weighs to be able to float.
the attractive force between two unlike substances
adhesion
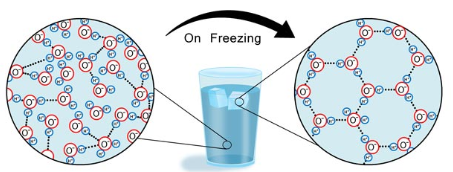
Why is ice less dense than liquid water?
ice molecules expand, causing more air pockets
the attractive force between two or more of the same molecules
cohesion
a property of liquid that allows it to resist external forces
surface tension
What allows water molecules to "stick" together?
cohesion
What allows this water strider to walk on water?
surface tension

What causes water droplets to stick to this spider web?
adhesion

What principle is this?
the buoyancy of a submerged object = the weight of the displaced liquid = weight of the object
Archimedes' Principle
What does it mean when an object has positive buoyancy?
the object will float
What does it mean when an object has neutral buoyancy?
the object will be suspende in liquid
What does it mean when an object has negative buoyancy?
the object will sink
the force a liquid exerts on a body in all directions at one depth
hydrostatic pressure
What causes decompression sickness?
When a diver goes up to the surface of the water to quickly at the end of a dive.
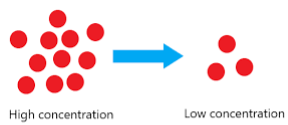
the process where matter in an area of high concentration moves to an area of low concentration
diffusion
When something becomes completely incorporated into a liquid.
dissolve

Does a lower temperature have more or less dissolved oxygen?
more dissolved oxygen
Does a higher temperature have more or less dissolved oxygen?
less dissolved oxygen
minerals and elements that serve a vital function in living organisms
nutrients
when two or more things work together to produce a greater impact, positive or negative, than either would have by itself
synergy
How acidic or basic something is
pH
Which mineral can make food taste different and corrode metal pipes
Chloride
Which mineral can stain your laundry and make water taste bitter
Iron
Which mineral an lower the dissolved oxygen in water, cause algae to rapidly reproduce, block hemoglobin in the blood from carrying oxygen to cells
Nitrogen
Which mineral can cause plants and protists to overly reproduce and lower the dissolved oxygen in water.
Phosphorus
Measurement of the amount of solids in a liquid that CAN pass through a 2-micron filter
Includes oxygen & nutrients
total dissolved solids (TDS)
Measurement of the amount of solids in a liquid that CANNOT pass through a 2-micron filter
Includes soil, trash & debris
total suspended solids (TSS)
Measurement the amount of light that is scattered by the solid particles
Turbidity
the settling of materials at the bottom of a liquid
Sedimentation

the settling of very small particles at the bottom of a liquid
Includes clay and sand
Considered a pollution factor
siltation

Bacteria found in the feces of humans and other animals
Can cause pollution & disease in water
Fecal Coliform





